-
 Taxes on super rich and tech giants stall under Trump
Taxes on super rich and tech giants stall under Trump
-
Star Wars series 'Andor' back for final season

-
 Neighbours improvise first aid for wounded in besieged Sudan city
Neighbours improvise first aid for wounded in besieged Sudan city
-
Tariffs could lift Boeing and Airbus plane prices even higher

-
 Analysts warn US could be handing chip market to China
Analysts warn US could be handing chip market to China
-
Unbeaten Miami edge Columbus in front of big MLS crowd in Cleveland

-
 Social media helps fuel growing 'sex tourism' in Japan
Social media helps fuel growing 'sex tourism' in Japan
-
'Pandora's box': alarm bells in Indonesia over rising military role

-
 Alaalatoa hails 'hustling hard' Brumbies for rare Super Rugby clean sheet
Alaalatoa hails 'hustling hard' Brumbies for rare Super Rugby clean sheet
-
Trio share lead at tight LA Championship

-
 Sampdoria fighting relegation disaster as old heroes ride into town
Sampdoria fighting relegation disaster as old heroes ride into town
-
Recovering pope expected to delight crowds at Easter Sunday mass

-
 Nuggets edge Clippers in NBA playoff overtime thriller, Knicks and Pacers win
Nuggets edge Clippers in NBA playoff overtime thriller, Knicks and Pacers win
-
Force skipper clueless about extra-time rules in pulsating Super Rugby draw

-
 Nuggets edge Clippers in NBA playoff overtime thriller, Pacers thump Bucks
Nuggets edge Clippers in NBA playoff overtime thriller, Pacers thump Bucks
-
Unbeaten Miami edge Columbus in front of big crowd in Cleveland

-
 Kim takes one-shot lead over Thomas, Novak at RBC Heritage
Kim takes one-shot lead over Thomas, Novak at RBC Heritage
-
Another round of anti-Trump protests hits US cities

-
 'So grateful' - Dodgers star Ohtani and wife welcome first child
'So grateful' - Dodgers star Ohtani and wife welcome first child
-
PSG maintain unbeaten Ligue 1 record, Marseille back up to second

-
 US, Iran report progress in nuclear talks, will meet again
US, Iran report progress in nuclear talks, will meet again
-
US Supreme Court intervenes to block Trump deportations

-
 Hamas armed wing says fate of US-Israeli captive unknown
Hamas armed wing says fate of US-Israeli captive unknown
-
Pacers thump Bucks to open NBA playoffs

-
 Sabalenka reaches Stuttgart semis as Ostapenko extends Swiatek mastery
Sabalenka reaches Stuttgart semis as Ostapenko extends Swiatek mastery
-
Zelensky says Ukraine will observe Putin's Easter truce but claims violations

-
 'Fuming' Watkins fires Villa in bid to prove Emery wrong
'Fuming' Watkins fires Villa in bid to prove Emery wrong
-
DR Congo boat fire toll revised down to 33
-
 England thrash Scotland to set up France Grand Slam showdown
England thrash Scotland to set up France Grand Slam showdown
-
Verstappen's Red Bull 'comes alive' to claim record pole in Jeddah

-
 McTominay fires Napoli level with Inter as Conte fuels exit rumours
McTominay fires Napoli level with Inter as Conte fuels exit rumours
-
Rajasthan unleash Suryavanshi, 14, as youngest IPL player but lose thriller

-
 Man City boost top five bid, Aston Villa thrash in-form Newcastle
Man City boost top five bid, Aston Villa thrash in-form Newcastle
-
Villa rout Newcastle to rekindle bid to reach Champions League

-
 Dumornay gives Lyon lead over Arsenal in Women's Champions League semis
Dumornay gives Lyon lead over Arsenal in Women's Champions League semis
-
Trans rights supporters rally in London, Edinburgh after landmark ruling

-
 'We have to wait': Barca's Flick on Lewandowski injury fear
'We have to wait': Barca's Flick on Lewandowski injury fear
-
Bordeaux-Begles backups edge Pau to close in on Top 14 summit

-
 Trans rights supporters rally outside in London, Edinburgh after landmark ruling
Trans rights supporters rally outside in London, Edinburgh after landmark ruling
-
PSG beat Le Havre to stay on course for unbeaten Ligue 1 season

-
 Man City close in on Champions League with Everton late show
Man City close in on Champions League with Everton late show
-
14-year-old Vaibhav Suryavanshi becomes youngest IPL player

-
 Barca make stunning comeback to beat Celta Vigo in Liga thriller
Barca make stunning comeback to beat Celta Vigo in Liga thriller
-
Zverev sets up birthday bash with Shelton in Munich

-
 Man City boost top five bid, Southampton snatch late leveller
Man City boost top five bid, Southampton snatch late leveller
-
US Supreme Court intervenes to pause Trump deportations

-
 Alcaraz and Rune race into Barcelona final
Alcaraz and Rune race into Barcelona final
-
US, Iran to hold more nuclear talks after latest round

-
 Man City close in on Champions League thanks to Everton late show
Man City close in on Champions League thanks to Everton late show
-
Bayern close in on Bundesliga title with Heidenheim thumping


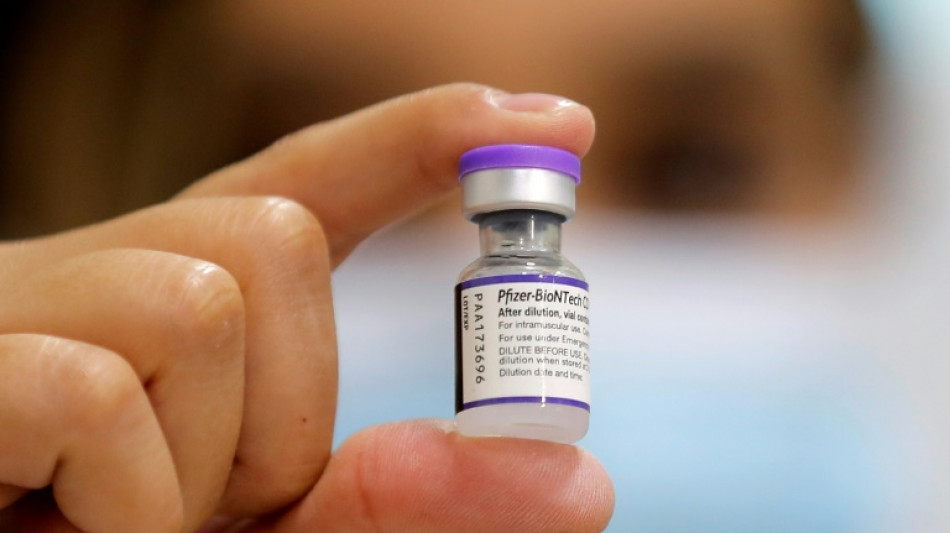
US drug regulator postpones meeting on infant Covid vaccines
The US Food and Drug Administration (FDA) on Friday announced it was pushing back a meeting scheduled for next week where a panel of experts would have decided whether to endorse the Pfizer-BioNTech Covid vaccine for infants.
The meeting would have evaluated whether two doses of the shot should be authorized for children aged six months through four years, even though clinical trials are still evaluating whether three shots are needed.
Interim results in fact had shown that a low dose of three micrograms given across this age group to stave off side effects did not elicit an immune response comparable to older age groups given a higher dose.
"Given that the study is advancing at a rapid pace, the companies will wait for the three-dose data as Pfizer and BioNTech continue to believe it may provide a higher level of protection in this age group," the US and German firms said in a statement.
"We will provide an update on timing for the advisory committee meeting once we receive additional data on a third dose in this age group from the company’s ongoing clinical trial and have an opportunity to complete an updated evaluation," added the FDA.
L.Dubois--BTB



