
-
 Less Soviet, more inspiring: Kyrgyzstan seeks new anthem
Less Soviet, more inspiring: Kyrgyzstan seeks new anthem
-
Defending champion Kyren Wilson crashes out in first round of World Snooker Championship

-
 NASA's oldest active astronaut returns to Earth on 70th birthday
NASA's oldest active astronaut returns to Earth on 70th birthday
-
Exec linked to Bangkok building collapse arrested

-
 Zelensky says Russian attacks ongoing despite Putin's Easter truce
Zelensky says Russian attacks ongoing despite Putin's Easter truce
-
Vaibhav Suryavanshi: the 14-year-old whose IPL dream came true

-
 Six drowning deaths as huge waves hit Australian coast
Six drowning deaths as huge waves hit Australian coast
-
Ukrainian soldiers' lovers kept waiting as war drags on

-
 T'Wolves dominate Lakers, Nuggets edge Clippers as NBA playoffs start
T'Wolves dominate Lakers, Nuggets edge Clippers as NBA playoffs start
-
Taxes on super rich and tech giants stall under Trump

-
 Star Wars series 'Andor' back for final season
Star Wars series 'Andor' back for final season
-
Neighbours improvise first aid for wounded in besieged Sudan city

-
 Tariffs could lift Boeing and Airbus plane prices even higher
Tariffs could lift Boeing and Airbus plane prices even higher
-
Analysts warn US could be handing chip market to China

-
 Unbeaten Miami edge Columbus in front of big MLS crowd in Cleveland
Unbeaten Miami edge Columbus in front of big MLS crowd in Cleveland
-
Social media helps fuel growing 'sex tourism' in Japan

-
 'Pandora's box': alarm bells in Indonesia over rising military role
'Pandora's box': alarm bells in Indonesia over rising military role
-
Alaalatoa hails 'hustling hard' Brumbies for rare Super Rugby clean sheet

-
 Trio share lead at tight LA Championship
Trio share lead at tight LA Championship
-
Sampdoria fighting relegation disaster as old heroes ride into town

-
 Recovering pope expected to delight crowds at Easter Sunday mass
Recovering pope expected to delight crowds at Easter Sunday mass
-
Nuggets edge Clippers in NBA playoff overtime thriller, Knicks and Pacers win

-
 Force skipper clueless about extra-time rules in pulsating Super Rugby draw
Force skipper clueless about extra-time rules in pulsating Super Rugby draw
-
Nuggets edge Clippers in NBA playoff overtime thriller, Pacers thump Bucks

-
 Unbeaten Miami edge Columbus in front of big crowd in Cleveland
Unbeaten Miami edge Columbus in front of big crowd in Cleveland
-
Kim takes one-shot lead over Thomas, Novak at RBC Heritage

-
 Another round of anti-Trump protests hits US cities
Another round of anti-Trump protests hits US cities
-
'So grateful' - Dodgers star Ohtani and wife welcome first child

-
 PSG maintain unbeaten Ligue 1 record, Marseille back up to second
PSG maintain unbeaten Ligue 1 record, Marseille back up to second
-
US, Iran report progress in nuclear talks, will meet again

-
 US Supreme Court intervenes to block Trump deportations
US Supreme Court intervenes to block Trump deportations
-
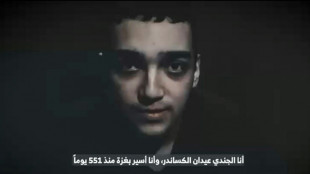
Hamas armed wing says fate of US-Israeli captive unknown
-
 Pacers thump Bucks to open NBA playoffs
Pacers thump Bucks to open NBA playoffs
-
Sabalenka reaches Stuttgart semis as Ostapenko extends Swiatek mastery

-
 Zelensky says Ukraine will observe Putin's Easter truce but claims violations
Zelensky says Ukraine will observe Putin's Easter truce but claims violations
-
'Fuming' Watkins fires Villa in bid to prove Emery wrong

-
 DR Congo boat fire toll revised down to 33
DR Congo boat fire toll revised down to 33
-
England thrash Scotland to set up France Grand Slam showdown

-
 Verstappen's Red Bull 'comes alive' to claim record pole in Jeddah
Verstappen's Red Bull 'comes alive' to claim record pole in Jeddah
-
McTominay fires Napoli level with Inter as Conte fuels exit rumours

-
 Rajasthan unleash Suryavanshi, 14, as youngest IPL player but lose thriller
Rajasthan unleash Suryavanshi, 14, as youngest IPL player but lose thriller
-
Man City boost top five bid, Aston Villa thrash in-form Newcastle

-
 Villa rout Newcastle to rekindle bid to reach Champions League
Villa rout Newcastle to rekindle bid to reach Champions League
-
Dumornay gives Lyon lead over Arsenal in Women's Champions League semis

-
 Trans rights supporters rally in London, Edinburgh after landmark ruling
Trans rights supporters rally in London, Edinburgh after landmark ruling
-
'We have to wait': Barca's Flick on Lewandowski injury fear

-
 Bordeaux-Begles backups edge Pau to close in on Top 14 summit
Bordeaux-Begles backups edge Pau to close in on Top 14 summit
-
Trans rights supporters rally outside in London, Edinburgh after landmark ruling

-
 PSG beat Le Havre to stay on course for unbeaten Ligue 1 season
PSG beat Le Havre to stay on course for unbeaten Ligue 1 season
-
Man City close in on Champions League with Everton late show


European medicines watchdog rejects new Alzheimer's drug
Europe's medicines watchdog on Friday rejected a marketing request for a new Alzheimer's disease treatment, saying the risks of the medicine's side effects, including potential brain bleeding, outweighed the benefits.
The decision by the Amsterdam-based European Medicines Agency was met with dismay, but experts said effective treatment for the degenerative mental disease affecting millions in Europe alone, was getting closer.
"The CHMP recommended not granting a marketing authorisation for Leqembi, a medicine intended for the treatment of Alzheimer's disease," the European Medicines Agency said, referring to its committee for evaluating drugs for human use.
Leqembi, which was developed by US multinational Biogen and Japanese-based Eisai, is the brand name of an active substance called lecanemab, which is used to treat adults with mild memory and cognitive problems resulting from the early stages of the common type of dementia.
But the CHMP said "the observed effect of Leqembi on delaying cognitive decline does not counterbalance the risk of serious side events associated with the medicine."
"The most important safety concern with Leqembi is the frequent occurrence of amyloid-related imaging abnormalities (ARIA), a side effect, seen in brain imaging, that involves swelling and potential bleedings in the brain," the EMA said.
- 'Unmet need' -
Leqembi is a monoclonal antibody, a type of protein that clings to a substance in the brain and can delay worsening of the disease. It is given intravenously every two weeks.
Leqembi, together with another Alzheimer's drug called Aduhelm -- also developed by Biogen and Eisai -- received approval from the US Food and Drug Administration (FDA) early last year.
Both drugs were approved through an accelerated process by the FDA for drugs treating serious conditions where there is an unmet medical need.
But in late January this year, Biogen pulled the controversial Aduhelm from the market, saying it was focusing on Leqembi instead.
Preliminary data from a trial of Leqembi was released in September 2022 and found it slowed cognitive decline in Alzheimer's patients by 27 percent.
Around eight million people in the European Union live with dementia, with Alzheimer's disease accounting for more than half of these cases, according to the Alzheimer Europe website.
Eisai, in a statement, said it was "extremely disappointed with the CHMP's negative opinion".
"There is a significant unmet need for new innovative treatment options that target an underlying cause of disease progression," Eisai's chief clinical officer Lynn Kramer said.
Eisai said it would seek a re-examination of the EMA's opinion "to ensure this treatment is available for eligible people living with early Alzheimer's disease in the EU as soon as possible."
The EMA said Eisai presented a main study involving 1,795 people with early Alzheimer's who either received Leqembi or a placebo, measured over a span of 18 months.
Eisai said that the watchdog added that its refusal had "no consequences for patients in clinical trials with Leqembi".
Eisai may now ask for a re-examination within 15 days, the EMA said.
- 'Ramp up efforts' -
Experts voiced disappointment at the EMA's refusal, but added there were "reasons to remain hopeful".
"Lecanemab has shown that it is possible to slow down disease progression, and research does work," said Tara Spires-Jones, president of the British Neuroscience Association.
"Now we need to ramp up our efforts to discover new and safer treatments," she said in a statement, adding that "each discovery brings us closer to new and better treatments."
Bart De Strooper, a professor in Alzheimer's disease at the University College London called the EMA's decision "unfortunate yet not unexpected".
"This conservative approach means that patients and doctors eager to explore a proven effective drug are now denied access," he said in a statement.
"With no current therapies available, it's disheartening to think that if we had applied such caution in the past, particularly with cancer drugs and their severe side effects, we might still be without cancer treatments today," De Strooper said.
H.Gerber--VB



