-
 Gold hits record, dollar drops as tariff fears dampen sentiment
Gold hits record, dollar drops as tariff fears dampen sentiment
-
As Dalai Lama approaches 90, Tibetans weigh future

-
 US defense chief shared sensitive information in second Signal chat: US media
US defense chief shared sensitive information in second Signal chat: US media
-
Swede Lingblad gets first win in just third LPGA start

-
 South Korea ex-president back in court for criminal trial
South Korea ex-president back in court for criminal trial
-
Thunder crush Grizzlies, Celtics and Cavs open NBA playoffs with wins

-
 Beijing slams 'appeasement' of US in trade deals that hurt China
Beijing slams 'appeasement' of US in trade deals that hurt China
-
Trump in his own words: 100 days of quotes

-
 Padres say slugger Arraez 'stable' after scary collision
Padres say slugger Arraez 'stable' after scary collision
-
Trump tariffs stunt US toy imports as sellers play for time

-
 El Salvador offers to swap US deportees with Venezuela
El Salvador offers to swap US deportees with Venezuela
-
Higgo holds on for win after Dahmen's late collapse

-
 El Salvador's president proposes prisoner exchange with Venezuela
El Salvador's president proposes prisoner exchange with Venezuela
-
Gilgeous-Alexander, Jokic, Antetokounmpo named NBA MVP finalists

-
 Thomas ends long wait with playoff win over Novak
Thomas ends long wait with playoff win over Novak
-
Thunder rumble to record win over Grizzlies, Celtics top Magic in NBA playoff openers

-
 Linesman hit by projectile as Saint-Etienne edge toward safety
Linesman hit by projectile as Saint-Etienne edge toward safety
-
Mallia guides Toulouse to Top 14 win over Stade Francais

-
 Israel cancels visas for French lawmakers
Israel cancels visas for French lawmakers
-
Russia and Ukraine trade blame over Easter truce, as Trump predicts 'deal'

-
 Valverde stunner saves Real Madrid title hopes against Bilbao
Valverde stunner saves Real Madrid title hopes against Bilbao
-
Ligue 1 derby interrupted after assistant referee hit by projectile

-
 Leclerc bags Ferrari first podium of the year
Leclerc bags Ferrari first podium of the year
-
Afro-Brazilian carnival celebrates cultural kinship in Lagos

-
 Ligue 1 derby halted after assistant referee hit by projectile
Ligue 1 derby halted after assistant referee hit by projectile
-
Thunder rumble with record win over Memphis in playoff opener

-
 Leverkusen held at Pauli to put Bayern on cusp of title
Leverkusen held at Pauli to put Bayern on cusp of title
-
Israel says Gaza medics' killing a 'mistake,' to dismiss commander

-
 Piastri power rules in Saudi as Max pays the penalty
Piastri power rules in Saudi as Max pays the penalty
-
Leaders Inter level with Napoli after falling to late Orsolini stunner at Bologna

-
 David rediscovers teeth as Chevalier loses some in nervy Lille win
David rediscovers teeth as Chevalier loses some in nervy Lille win
-
Piastri wins Saudi Arabian Grand Prix, Verstappen second

-
 Kohli, Rohit star as Bengaluru and Mumbai win in IPL
Kohli, Rohit star as Bengaluru and Mumbai win in IPL
-
Guirassy helps Dortmund past Gladbach, putting top-four in sight

-
 Alexander-Arnold lauds 'special' Liverpool moments
Alexander-Arnold lauds 'special' Liverpool moments
-
Pina strikes twice as Barca rout Chelsea in Champions League semi

-
 Rohit, Suryakumar on song as Mumbai hammer Chennai in IPL
Rohit, Suryakumar on song as Mumbai hammer Chennai in IPL
-
Dortmund beat Gladbach to keep top-four hopes alive

-
 Leicester relegated from the Premier League as Liverpool close in on title
Leicester relegated from the Premier League as Liverpool close in on title
-
Alexander-Arnold fires Liverpool to brink of title, Leicester relegated

-
 Maresca leaves celebrations to players after Chelsea sink Fulham
Maresca leaves celebrations to players after Chelsea sink Fulham
-
Trump eyes gutting US diplomacy in Africa, cutting soft power: draft plan

-
 Turkey bans elective C-sections at private medical centres
Turkey bans elective C-sections at private medical centres
-
Lebanon army says 3 troops killed in munitions blast in south

-
 N.America moviegoers embrace 'Sinners' on Easter weekend
N.America moviegoers embrace 'Sinners' on Easter weekend
-
Man Utd 'lack a lot' admits Amorim after Wolves loss

-
 Arteta hopes Arsenal star Saka will be fit to face PSG
Arteta hopes Arsenal star Saka will be fit to face PSG
-
Ukrainian troops celebrate Easter as blasts punctuate Putin's truce

-
 Rune defeats Alcaraz to win Barcelona Open
Rune defeats Alcaraz to win Barcelona Open
-
Outsider Skjelmose in Amstel Gold heist ahead of Pogacar and Evenepoel


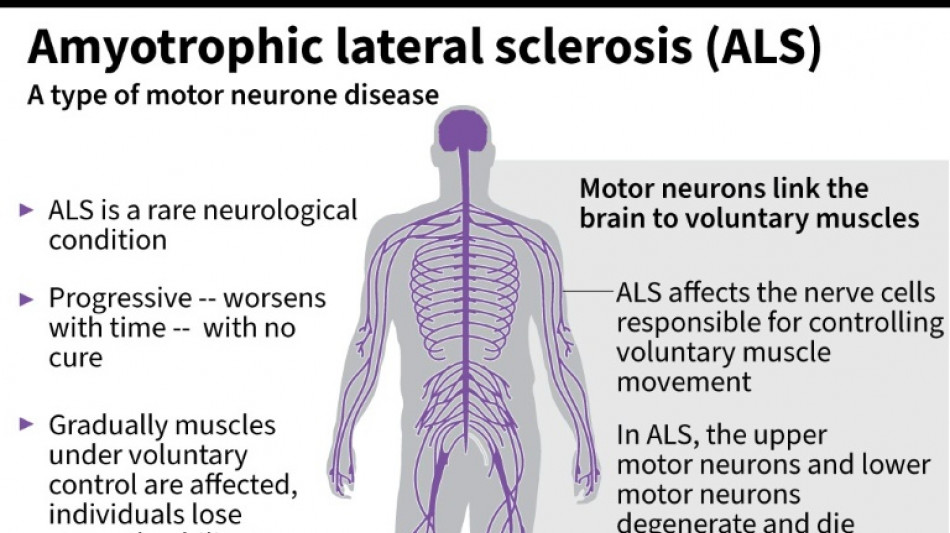
US company withdraws ALS drug after it fails in trial
Amylyx Pharmaceuticals announced Thursday it was withdrawing its approved treatment against the deadly neurodegenerative disease ALS after clinical data found no evidence the drug worked.
In a statement, the US company said it would discontinue its market authorizations for Relyvrio/Albrioza, using the brand names of the medicine in the US and Canadian markets.
"While this is a difficult moment for the ALS community, we reached this path forward in partnership with the stakeholders who will be impacted and in line with our steadfast commitment to people living with ALS and other neurodegenerative diseases," said the company's co-CEOs Joshua Cohen and Justin Klee in a statement.
The company also said it was reducing its workforce "by approximately 70 percent" as it focused on another experimental drug for use against ALS, and on repurposing Relyvrio for other conditions. It added it would continue to make Relyvrio available for patients who wish to keep using the treatment, through a "free drug program."
The news follows data from a clinical trial of 664 ALS patients announced in March, which found no significant differences in outcomes between those on the treatment group and those who received a placebo.
It was a big blow for patients with amyotrophic lateral sclerosis, sometimes called Lou Gehrig's disease after the famous baseball player, which devastates nerve cells in the brain and spinal cord.
ALS affects about two people per 100,000 every year, causing progressive loss of motor and cognitive function. Most patients die within five years of their diagnosis.
Relyvrio's approval by the US Food and Drug Administration in 2022 was controversial and based on the results of a single trial that involved just 137 participants.
The FDA itself noted there was "residual uncertainty about the evidence of effectiveness" -- but "given the serious and life-threatening nature of ALS and the substantial unmet need, this level of uncertainty is acceptable in this instance and consideration of these results in the context of regulatory flexibility is appropriate."
- Patient groups backed approval -
Advocacy groups also mounted a major campaign sending a petition to the FDA with tens of thousands of signatures urging approval. Once it became available, Amylyx reportedly announced an eye-watering list price of $158,000 per year in the US, drawing criticism.
Patient groups in Europe watched with desperation at the bureaucratic delays.
When the European Union drug watchdog later announced it was rejecting Relyvrio, the decision was slammed as "an affront" by angry French patients, who say they "don't have time to wait." France later relented, offering conditional approval in November.
"We commend Amylyx for pulling Relyvrio off the market, while still ensuring that people living with ALS can access the drug if they believe it is helping them," said the US-based ALS association, which had lobbied for the drug's approval and funded its research.
"Safe and potentially effective treatments can be made accessible rapidly until further research can confirm their efficacy," it added.
For now, there remain only a handful of treatments available.
Riluzole, FDA approved in 1995, prolongs life approximately three months. Edaravone, FDA approved in 2017, has been found to slow disease progression and improve survival.
And in 2023, the regulatory body approved tofersen, a gene therapy treatment that targets those ALS cases that are caused by mutations in the SOD1 gene.
C.Stoecklin--VB




