
-
 Pentagon chief dismisses reports he shared military info with wife
Pentagon chief dismisses reports he shared military info with wife
-
15 potential successors to Pope Francis

-
 The papabili - 15 potential successors to Pope Francis
The papabili - 15 potential successors to Pope Francis
-
Zhao sets up all-China clash after beating 2024 world snooker finalist Jones

-
 Ostapenko stuns Sabalenka to win Stuttgart title
Ostapenko stuns Sabalenka to win Stuttgart title
-
Argentina mourns loss of papal son

-
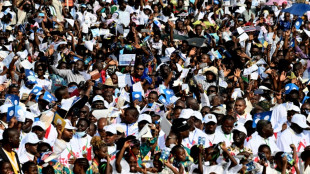 African leaders praise Pope Francis's 'legacy of compassion'
African leaders praise Pope Francis's 'legacy of compassion'
-
Mehidy's five wickets help Bangladesh fight back in first Zimbabwe Test

-
 'The voice of god': Filipinos wrestle with death of Pope Francis
'The voice of god': Filipinos wrestle with death of Pope Francis
-
Prayers, disbelief in East Timor after Pope Francis death

-
 Real Madrid hold minute's silence as La Liga mourns Pope Francis
Real Madrid hold minute's silence as La Liga mourns Pope Francis
-
World leaders pay tribute to Pope Francis, dead at 88

-
 World leaders react to the death of Pope Francis
World leaders react to the death of Pope Francis
-
Zimbabwe lead first Test despite Bangladesh spinner Mehidy's five wickets

-
 Vatican postpones sainthood for 'God's influencer' after pope's death
Vatican postpones sainthood for 'God's influencer' after pope's death
-
Pope's death prompts CONI to call for sporting postponements, minute's silence

-
 Stunned and sad, faithful gather at St Peter's to remember Francis
Stunned and sad, faithful gather at St Peter's to remember Francis
-
Asian scam centre crime gangs expanding worldwide: UN

-
 Davos meet founder Klaus Schwab steps down from WEF board
Davos meet founder Klaus Schwab steps down from WEF board
-
Himalayan snow at 23-year low, threatening 2 billion people: report

-
 The beautiful game: Pope Francis's passion for football
The beautiful game: Pope Francis's passion for football
-
Clerical sex abuse: Pope Francis's thorniest challenge

-
 Pope Francis's delicate ties with politics in Argentina
Pope Francis's delicate ties with politics in Argentina
-
Russia resumes attacks on Ukraine after Easter truce

-
 Pope Francis has died aged 88
Pope Francis has died aged 88
-
Gaza civil defence describes medic killings as 'summary executions'

-
 Francis: radical leader who broke the papal mould
Francis: radical leader who broke the papal mould
-
Oscar stars, Max keeps mum, Sainz alive - Saudi GP talking points

-
 Iyer, Kishan win back India contracts as Pant's deal upgraded
Iyer, Kishan win back India contracts as Pant's deal upgraded
-
Vance lands in India for tough talks on trade

-
 Inside South Africa's wildlife CSI school helping to catch poachers
Inside South Africa's wildlife CSI school helping to catch poachers
-
Nigerian Afrobeat legend Femi Kuti takes a look inward

-
 Kim Kardashian: From sex tape to Oval Office via TV and Instagram
Kim Kardashian: From sex tape to Oval Office via TV and Instagram
-
Vance in India for tough talks on trade

-
 Thunder crush Grizzlies as Celtics, Cavs and Warriors win
Thunder crush Grizzlies as Celtics, Cavs and Warriors win
-
Vance heads to India for tough talks on trade

-
 China slams 'appeasement' of US as nations rush to secure trade deals
China slams 'appeasement' of US as nations rush to secure trade deals
-
'Grandpa robbers' go on trial for Kardashian heist in Paris

-
 Swede Lindblad gets first win in just third LPGA start
Swede Lindblad gets first win in just third LPGA start
-
Gold hits record, dollar drops as tariff fears dampen sentiment

-
 As Dalai Lama approaches 90, Tibetans weigh future
As Dalai Lama approaches 90, Tibetans weigh future
-
US defense chief shared sensitive information in second Signal chat: US media

-
 Swede Lingblad gets first win in just third LPGA start
Swede Lingblad gets first win in just third LPGA start
-
South Korea ex-president back in court for criminal trial

-
 Thunder crush Grizzlies, Celtics and Cavs open NBA playoffs with wins
Thunder crush Grizzlies, Celtics and Cavs open NBA playoffs with wins
-
Beijing slams 'appeasement' of US in trade deals that hurt China

-
 Trump in his own words: 100 days of quotes
Trump in his own words: 100 days of quotes
-
Padres say slugger Arraez 'stable' after scary collision

-
 Trump tariffs stunt US toy imports as sellers play for time
Trump tariffs stunt US toy imports as sellers play for time
-
El Salvador offers to swap US deportees with Venezuela

| RBGPF | 0% | 63.45 | $ | |
| RYCEF | -0.96% | 9.41 | $ | |
| CMSD | -0.78% | 21.79 | $ | |
| SCS | -3.61% | 9.42 | $ | |
| NGG | 0.58% | 72.53 | $ | |
| RIO | -0.02% | 58.16 | $ | |
| GSK | 0.51% | 36.115 | $ | |
| RELX | -0.13% | 52.13 | $ | |
| VOD | -0.49% | 9.265 | $ | |
| CMSC | -0.37% | 21.74 | $ | |
| AZN | -0.07% | 67.545 | $ | |
| JRI | -0.5% | 12.338 | $ | |
| BCC | -2.94% | 90.8 | $ | |
| BCE | 0.29% | 22.105 | $ | |
| BP | -2.26% | 27.695 | $ | |
| BTI | 0.26% | 42.48 | $ |

Biogen pulls controversial Alzheimer's drug Aduhelm
A controversial Alzheimer's drug that was trumpeted as the first to ever treat the cognitive decline associated with the devastating brain disorder has been pulled from the market, its maker Biogen announced Wednesday.
The US Food and Drug Administration awarded accelerated approval to Aduhelm in June 2021, a decision that was highly contentious at the time because the agency overruled its own independent advisors, who found there was insufficient evidence of benefit.
At least three of the 11-member independent committee that voted unanimously against recommending the drug subsequently resigned, and US congressional investigators slammed the accelerated approval as "rife with irregularities."
Biogen said it was discontinuing Aduhelm to put more resources into Leqembi, a newer Alzheimer's medicine that was fully approved last year under the traditional regulatory pathway.
"When searching for new medicines, one breakthrough can be the foundation that triggers future medicines to be developed," said Christopher Viehbacher, president and CEO of the Cambridge, Massachusetts-based biotech firm.
"Aduhelm was that groundbreaking discovery that paved the way for a new class of drugs and reinvigorated investments in the field."
Aduhelm, a monoclonal antibody that targets the build-up of a protein called amyloid beta in the brain tissue which is thought to be a cause of Alzheimer's, was tested in two late-stage human trials.
It showed a reduction in cognitive decline in one of the studies, but not the other.
According to a congressional report from December 2022, the FDA "considered Aduhelm under the traditional approval pathway used for most drugs for nine months, before abruptly changing course and granting approval under the accelerated approval pathway after a three-week review period."
The report said that FDA interactions with Biogen were "atypical" and included a failure to properly document contacts between agency staff and the drug maker.
The FDA and Biogen had also "inappropriately collaborated" on a joint briefing document for a key advisory committee, it said.
"FDA's approval process was rife with irregularities."
As for Biogen, the report said the company "viewed Aduhelm as an unprecedented financial opportunity -- estimating a potential peak revenue of $18 billion per year."
The congressional panel pointed to an "unjustifiably high price" for Aduhelm of $56,000 a year for patients.
Biogen's Leqembi, which it co-manufactures with Eisai of Japan, is now the only US approved treatment for Alzheimer's. It also targets amyloid beta and has been found to modestly reduce cognitive decline in patients with early stage disease.
Donanemab, developed by Eli Lilly, could be next to get the green light after performing similarly in clinical trials.
Alzheimer's is the most common form of dementia. More than one in nine people over 65 develop the condition, which worsens over time, robbing them of their memories and independence, according to the US Alzheimer's Association.
S.Leonhard--VB




